Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.75P
Related questions
Question
- A chemist was charged with the task of making amino alcohol 2. This chemist decided to attempt a Grignard reaction between two equivalents of phenylmagnesium bromide and amino ester 1. To this end, two equivalents of the Grignard reagent were added to a flask containing a stirred THF solution of ester 1 at -78 °C. Upon acidic aqueous work-up of the reaction mixture, the desired alcohol 2 was not isolated, even though one equivalent of methanol was produced and all the starting materials were consumed. Instead, two products with the molecular formulae of C6H6 and C7H13NO were obtained in a 2:1 ratio, respectively. Identify the structures of these two products and describe, mechanistically, how they were formed.
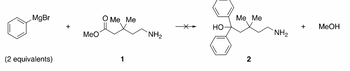
Transcribed Image Text:MgBr
MeO
O Me Me
(2 equivalents)
1
Me Me
+
MeOH
но
NH2
NH2
2
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning